STELLAR-1
A clinical study for children with SMA
The STELLAR-1 study is for children under 6 weeks old who have been genetically diagnosed with Spinal Muscular Atrophy (SMA) but are not yet showing symptoms (presymptomatic). It evaluates an investigational drug called salanersen.
Important: To give your child the opportunity to take part in this study, screening ideally should happen before they turn 4 weeks old.

About the STELLAR-1 study
Evaluating an investigational drug for SMA
The STELLAR-1 Study is evaluating the effect salanersen has on infants diagnosed with spinal muscular atrophy (SMA) through genetic testing.
If you are interested in having your child join the STELLAR-1 Study, they must:
- Be under 6 weeks old
- Have a genetic diagnosis of SMA, with either 2 or 3 copies of the SMN2 gene
- Have not experienced any symptoms of SMA before receiving the study drug
- Have not received any other treatment for SMA
There are more requirements to join the study, which will be explained by the study team.
What causes SMA?
SMA is a rare, genetic disease that affects motor neurons. These are the nerves that control muscle movement. SMA weakens or destroys motor neurons, causing muscles to waste away. Over time, this can lead to problems with moving, breathing, and swallowing. SMA affects each person differently––some may notice motor function changes slowly, while others may see them more quickly.
In most people living with SMA, changes in a gene called survival motor neuron 1 (SMN1) – often referred to as gene mutations or variants – affect how this gene works. As a result, their bodies produce less SMN protein. There is another SMN2 gene that produces SMN protein, but it usually does not produce enough SMN protein on its own to make up for the changes in the SMN1 gene.

What is salanersen?
The study drug being evaluated in the STELLAR-1 Study is called salanersen.
In people with SMA, changes in the SMN1 gene lower the overall amount of SMN protein in the body. Without enough of this protein, motor neurons and muscles lose their ability to work properly. A similar gene called SMN2 can help replace some of the lost SMN protein in the body. Salanersen is designed to help the SMN2 gene to make more SMN protein.
LP is also used to collect small samples of the fluid around the spine (called cerebrospinal fluid). These samples allow the study team to monitor the level of salanersen in your body and perform other safety tests.
Salanersen has been tested in a Phase 1 clinical research study for participants aged six months to 12 years old. Researchers want to see if taking salanersen before symptoms appear improves outcomes for babies with SMA. Ideally, study screening should happen before they turn 4 weeks old.
Regardless of whether you join the clinical study or not, it is important to work with your child’s doctor about their care plan as soon as possible.
How it works
You're not alone.
Here's how to get started.
As the STELLAR-1 study is for children under 6 weeks old, timing is very important. We are here to guide you through the process.
1
Share your details
Fill out a short form so we can connect you with a Patient Navigator.- Our Patient Navigators are here to support you throughout the process of determining whether your child may qualify for the STELLAR-1 study. They will also help connect you with a study site promptly.
- Participation is entirely voluntary, and you may choose to withdraw at any time.
- Your information is kept private
2
Quick eligibility check
Answer a few brief questions about your child’s health and diagnosis.- We will only ask for the minimum medical information needed
- You will receive clear next steps based on your responses
3
Talk with your navigator
Book a free 30-minute call with a dedicated Patient Navigator.- They will explain the basics of the study, answer your initial questions, and guide you through next steps
- If your conversation with the Patient Navigator indicates that your child may qualify, they will connect you to a study site
- Support will be offered in your language, with interpreters provided if needed
Frequently asked questions
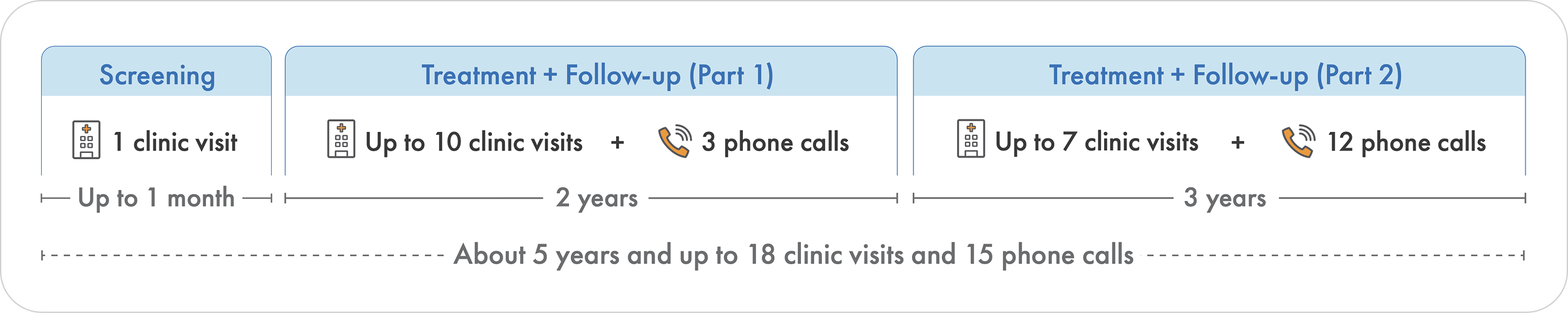
How long is the study?
The total study duration is about 5 years long. The study includes two parts: Part 1 and Part 2. Part 1 lasts about 2 years, during which your child will receive two doses of the study drug, salanersen. Each dose is given about 12 months apart. Part 1 includes a screening period, 10 clinic visits, and up to 3 phone check-ins. After completing Part 1, your child will continue to Part 2, during which your child will receive three additional doses of salanersen about 12 months apart, for about 3 years. During Part 2, there will be up to 7 clinic visits and 12 phone check-ins.
The study team will walk you through the full timeline and answer any questions you may have. Your child’s participation is completely voluntary, and you can withdraw at any time.
Will there be any reimbursement and/or travel support?
Yes. Travel reimbursement is available to you and your family to support your participation in the STELLAR-1 Study. There is no cost to families for the study drug, study-related care, or monitoring.
What should we expect during the study?
Before your child can join the study, there is a screening process with tests and checks to confirm eligibility. If eligible, your child will receive two doses of the study drug in Part 1 and up to three more yearly doses in Part 2.
There will be regular clinic visits to monitor effects such as motor skills, growth, breathing, and overall health. Some visits may require an overnight stay. Blood, urine, and spinal fluid samples will also be collected to ensure safety and understand how the study drug works. Participants will also undergo physical and neurological exams, as well as electrocardiograms (ECGs).
What kind of care will my child receive during the study?
Your child can usually continue seeing their regular healthcare providers while participating in the study.
At the same time, the clinical study offers access to a dedicated study team who carefully monitors your child’s health and progress. This team focuses on the condition being studied and provides all study related medications and procedures at no cost. The study does not replace your child’s overall medical care but adds an extra layer of support.
What support or follow-up care is available after the study ends?
The study team will help you plan the next steps and share all study-related health information with your child’s regular doctor to help with continuity of care.
How long is the study?
The total study duration is about 5 years long. The study includes two parts: Part 1 and Part 2. Part 1 lasts about 2 years, during which your child will receive two doses of the study medicine, salanersen. Each dose is given about 12 months apart. Part 1 includes a screening period, 10 clinic visits, and up to 3 phone check-ins. After completing Part 1, your child will continue to Part 2, during which your child will receive three additional doses of salanersen about 12 months apart, for about 3 years. During Part 2, there will be up to 7 clinic visits and 12 phone check-ins.
The study team will walk you through the full timeline and answer any questions you may have. Your child’s participation is completely voluntary, and you can withdraw at any time.
Will there be any reimbursement and/or travel support?
Yes. Travel reimbursement is available to you and your family to support your participation in the STELLAR-1 Study. There is no cost to families for the study medicine, study-related care, or monitoring.
What should we expect during the study?
Before your child can join the study, there is a screening process with tests and checks to confirm eligibility. If eligible, your child will receive two doses of the study medicine in Part 1 and up to three more yearly doses in Part 2.
There will be regular clinic visits to monitor effects such as motor skills, growth, breathing, and overall health. Some visits may require an overnight stay. Blood, urine and spinal fluid samples will also be collected to ensure safety and to understand how the study medicine works. Participants will also undergo physical and neurological examinations, as well as electrocardiograms (ECGs).
What kind of care will my child receive during the study?
Your child can usually continue seeing their regular healthcare providers while participating in the study.
At the same time, the clinical study offers access to a dedicated study team who carefully monitors your child’s health and progress. This team focuses on the condition being studied and provides all study related medications and procedures at no cost. The study does not replace your child’s overall medical care but adds an extra layer of support.
What support or follow-up care is available after the study ends?
The study team will help you plan the next steps and share all study-related health information with your child’s regular doctor to help with continuity of care.
Find a study near you
STELLAR-1 is a global study running at hospitals and clinics in 18 countries around the world.
Your Patient Navigator will work with you to connect to the right site and answer any questions about travel or logistics.
See available study sites in the US on the map below:
Please read, your consent is required
If you are a parent or caregiver of a child with genetically diagnosed Spinal Muscular Atrophy (SMA), you are invited to complete pre-screening questions to determine whether your child may be eligible for participation in this SMA clinical study.
Biogen, the sponsor of this study, is responsible for the protection of your child’s personal information. Biogen has engaged myTomorrows (“myTomorrows”, “we” or “us”) to support study recruitment for the study by collecting pre-screening information for the study through this website.
With your consent, we will use this information to evaluate your child’s potential eligibility for the study.
The information that could identify your child will not be shared with Biogen. Only de-identified data will be shared with Biogen MA Inc.
You may withdraw your consent at any time and upon withdrawal of your consent we will promptly delete your child’s personal data, except to the extent required to comply with our legal obligations. If you have a question, wish to withdraw consent, or exercise your other privacy rights, please contact myTomorrows at dataprotection@mytomorrows.com as Biogen MA Inc. will not receive any information that can directly identify your or your child. For more information about the Biogen Privacy Policy, please visit https://www.biogen.com/privacy-center.html or https://www.biogen.com/privacy-center/international-privacy.html.
After answering the pre-screener questions, you will have the opportunity to schedule a call with one of our Patient Navigators, who will collect additional information about your child’s health to assess the initial eligibility for this study. If potentially eligible, they will provide you with further information and answer questions about possible site locations, travel logistics and preferences, if applicable. If we find that your child may be eligible for this study, and you confirm your interest in participating, we will, with your consent, share the personal information collected with a clinical study site. The site will contact you to discuss next steps and answer any questions you may have. Your child’s information may also be shared with other vendors of the Sponsor.
Even if your child does not qualify for this study, it is possible to schedule a call with one of our Patient Navigators to discuss other options that may be available to your child.
We will retain your child’s data for 2 years so we can get in touch with you if other options become available to your child during this time period.


