A Clinical Study of ABO-101 for Patients with Primary Hyperoxaluria Type 1


If you have PH1 and wish to participate in a clinical trial, you may be able to take part in the redePHine study regardless of where you are in the world.
The redePHine study is looking for individuals with Primary Hyperoxaluria Type 1 (PH1) to participate in a clinical research study of a new gene editing therapy.
ABO-101 is an investigational medicine or study drug and is a potential one-time gene editing infusion, aiming to lower your levels of oxalate. Since PH1 leads to high oxalate levels, reducing these levels may help manage the symptoms of this condition.


Share your contact details to allow us to get in touch with you.

Complete a questionnaire with some first initial checks to assess if you may potentially qualify for the redePHine study. This questionnaire will only gather the minimum medical data needed to assess your potential eligibility for the study.

If you seem potentially eligible based on the pre-screening questionnaire answers, you can schedule a free call with a Patient Navigator. Your Patient Navigator will further assess your potential qualification for the redePHine study and provide you with the information and assistance you need to be referred to the study site, to determine your final eligibility.
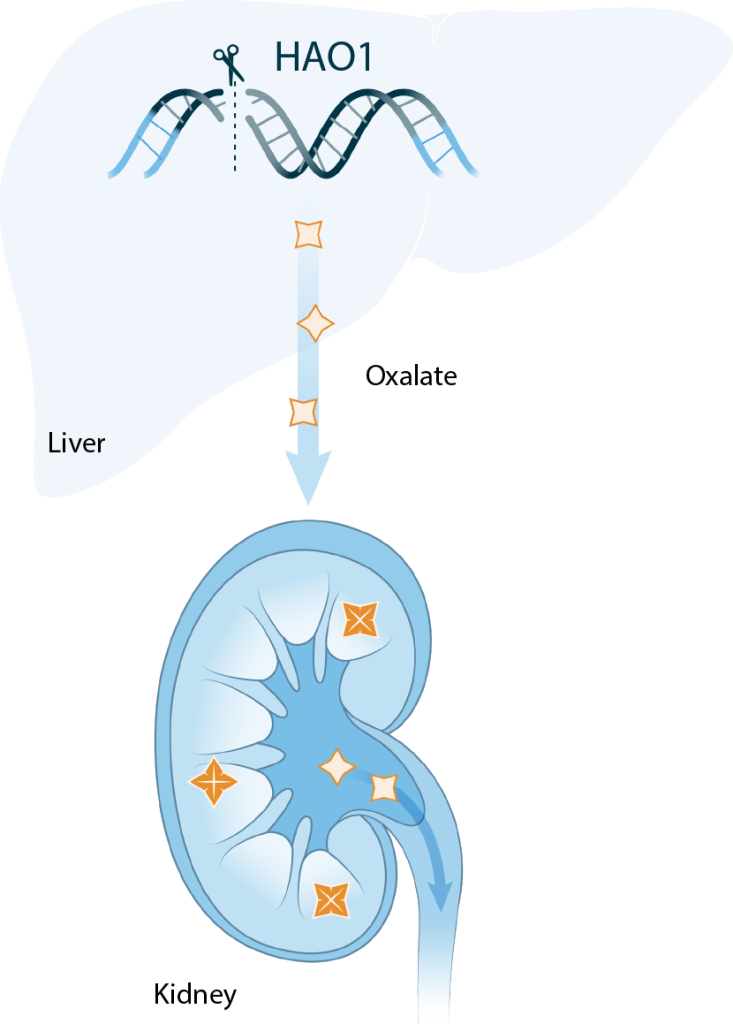
Primary Hyperoxaluria Type 1 (PH1) is a rare genetic condition caused by a mutation (change in the DNA) within the AGXT gene. This mutation leads to the liver not producing enough of a special protein, resulting in high levels of oxalate. Normally, oxalate can be processed and eliminated by the kidneys. However, when oxalate levels become too high, it can form crystals that may cause stones in the urinary tract and in the kidneys. Symptoms may include pain when urinating, blood in urine and urinary tract infections. Chronic formation of stones can lead to kidney disease and potentially progress to kidney failure.
If kidney function declines significantly, oxalate can build up in other parts of the body, leading to crystal formation in areas such as the eyes, skin, or heart.
PH1 is an inherited condition, meaning that it can be passed down in families.
Current management options aim to reduce crystal formation caused by oxalate overproduction, helping to slow down kidney damage and decrease stone formation. Your doctor may recommend drinking a lot of fluids, making dietary changes, and in some cases taking vitamin B6 (pyridoxine). When available, your doctor may prescribe medicines to lower oxalate levels in urine (for example, lumasiran [Oxlumo™] or nedosiran [Rivfloza™]). If kidney stones cause pain or block urine flow, they may need to be removed or broken down so they can pass in the urine. In some people with PH1, their kidneys may work less effectively over time. In those cases, a treatment called dialysis may be recommended.
In certain cases, liver transplant, with or without kidney transplant may be considered curative for PH1. However, this is a significant surgical procedure with notable risks, including the need for lifelong immunosuppression, and it is only indicated in some patients.
Research into new treatments for PH1 is ongoing.
Gene editing is a type of genetic medicine that can directly change individual genes to treat a disease.
One of the most researched methods is called CRISPR. This tool can find and precisely edit specific genes.
ABO-101 is being studied as a potential one-time CRISPR gene-editing therapy.
Gene editing is approved by the Food and Drug Administration (FDA) in the U.S. for the treatment of sickle cell disease, while in Europe, the European Medicines Agency (EMA) has approved it for both sickle cell disease and beta thalassemia.
Gene editing is also being investigated in other regions for transthyretin amyloidosis (a rare heart and nerve disease), hypercholesterolemia (high cholesterol), and immune disorders.
Clinical trials are clinical research studies that evaluate the safety, tolerability, and effectiveness of investigational therapies. They play a crucial role in developing treatments with the aim of improving patient health. Watch our video to learn more.
Clinical trials are essential to ensure treatments are safe for the public, determine optimal administration methods, and enhance scientific knowledge of diseases for better health outcomes.
The term ‘investigational medicine’ or ‘study drug’ refers to a trial medication that has not yet received approval from regulatory authorities, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). As a result, it can only be utilized in clinical research studies and is not available for general use. These studies aim to evaluate the safety and efficacy of an investigational medicine before it can potentially become approved for widespread medical use.
Clinical trials have specific criteria, known as clinical trial eligibility criteria, which determine who can participate. These criteria are designed to identify individuals with specific symptoms or a particular diagnosis, while excluding those for whom trial participation would be inappropriate. By enrolling participants who are similar in their characteristics, such as the type of mutation and disease progression, clinical trial eligibility criteria ensure that the outcomes of the trial accurately reflect the effects of the investigational drug being evaluated. Furthermore, these criteria play a crucial role in ensuring the safety of the participants involved.
Clinical trials carry potential risks like treatment side effects, unknown treatment effectiveness, extra medical tests, and time commitments. These are carefully assessed and monitored, and risks and benefits are weighed by the Ethics Committees reviewing and approving the trial’s conduct. Clinical trials follow a specific set of standards and are closely regulated to help ensure the safety of all participants. Safety measures and informed consent processes help participants understand and manage these risks.
People take part in clinical trials for different reasons. Some may take part because they want to learn more about their disease and potential new treatments. Others volunteer to take part because they want to help researchers learn more about (the treatment of) a disease to potentially help them and others in the future.
Yes. Participants receive detailed trial information, can ask questions, and decide freely. Informed consent ensures they understand the trial and can withdraw at any time without affecting their care or rights.
At myTomorrows, we assign a dedicated Patient Navigator to each patient. Your Patient Navigator will provide comprehensive support throughout the entire process, from assessing your trial eligibility to addressing any questions you may have during your clinical trial journey. Our Patient Navigators have a medical background and are closely supervised by our team of medical doctors. While they are trained to explain complex medical concepts, they cannot give medical advice. Our Patient Navigator team offers dependable, multilingual support covering all time zones, ensuring effective communication and understanding with patients from diverse backgrounds.
Yes, besides servicing pre-screening for specific clinical trials, myTomorrows also offers other free of charge services regarding providing unbiased information about clinical trials or other pre-approval treatment options and how to access them. This is always free of charge.
The purpose of this study is to evaluate the safety, tolerability, and preliminary efficacy of an investigational gene editing drug in people with Primary Hyperoxaluria Type 1 (PH1).
This is a dose escalation study, which means that it will involve testing several different doses of the medication. Initial adult participants will receive a low dose. If that dose is well-tolerated, the next group of participants will receive an intermediate dose, and if that dose is also well-tolerated, a higher dose will be given to the following group. This process helps us determine the safest and most effective dose. Once we identify a safe and effective dose in adults, all subsequent participants will receive this dose, including pediatric patients.
ABO-101, a potential one-time CRISPR gene editing investigational treatment, is the study drug being evaluated in the redePHine study.
ABO-101 is a potential one-time CRISPR gene editing investigational therapy designed to specifically edit the HAO1 gene to permanently stop it from working. Previous PH1 clinical studies have shown that inhibiting HAO1 results in lower oxalate levels in people with PH1. Since PH1 leads to high levels of oxalate, reducing these levels may help manage symptoms of this condition and lower the chance of kidney stones and kidney damage.
Yes, all participants in the clinical research study will receive investigational therapy. There is no placebo dose in this study.
Adult participants may receive a low, medium, or high dose of the drug. Once we identify a safe and effective dose in adults, all subsequent participants will receive this investigational dose, including pediatric patients.
Eligible participants must:
There are additional requirements, which the study doctor will discuss with potentially eligible individuals interested in participating.
If you may potentially qualify, you can be connected to the study site closest to you to minimize travel and burden. However, if you do need to travel nationally or internationally, reimbursement for expenses will be provided to facilitate your participation. The study site will work with you to ensure you receive this.
For the first phase of the study, you will receive a single dose of the study drug (ABO- 101) and your health will be closely monitored for the first 2 years. Following that, there will be a long-term follow-up period lasting up to 13 years post-study drug (ABO-101) administration to study the continued safety and efficacy of this new gene editing therapy.
This study will include:
If there is no study site near you, you will still be able to participate in this study. Travel and reimbursement for expenses will be arranged to facilitate your participation. The study site will work with you to ensure you receive this.

 Study centers grouped together (> 10)
Study centers grouped together (> 10)
 Study centers grouped together (< 10)
Study centers grouped together (< 10)
 Study centers recruiting
Study centers recruiting
 Study centers preparing to recruit
Study centers preparing to recruit
The following pages contain some pre-screening questions to determine whether you (the patient) may be eligible to take part in the redePHine clinical research study. If you would like to participate in the pre-screening, you will need to provide certain personal information and information about your health. If you’re answering the questions on behalf of the patient, please fill in your personal contact information and answer any medical question ‘as the patient’.
Arbor Biotechnologies, Inc., is the sponsor of this study and is responsible for ensuring the security and confidentiality of your personal information in accordance with all applicable data protection regulations and can be contacted at privacy@arbor.bio. Arbor Biotechnologies has engaged myTomorrows (“myTomorrows”, “we” or “us”) and Worldwide Clinical Trials to assist with clinical trial recruitment for the study by collecting pre-screening information for the study through this website.
We will use this information, with your consent, to evaluate your potential eligibility for the study.
After answering the pre-screening questions, you will have the opportunity to schedule a call with one of our Patient Navigators, who will collect additional information about your health to assess your eligibility for this trial and information about your travel possibilities and preferences to support your referral to a clinical trial site, should you be eligible for the study. If we find that you may be eligible for this study, we will share your personal information with a clinical trial site, who may share your personal information with Scout Clinical, another vendor of Arbor Biotechnologies, if needed to support you with travel arrangements.

If we find that you may be eligible for the trial, your data may be transferred and stored internationally, including to countries where the data protection laws may be different than in the country where we collect your personal information. However, the sponsor is required to ensure that adequate security and organizational control procedures are implemented by any party, including myTomorrows, that may access your personal information to ensure that your personal information will continue to be protected at the same level as in the country where it is collected.
We will store any information you provide through this website for as long as necessary to fulfill the purposes listed above, in accordance with applicable laws, or for a period of two years if you are not eligible and elect for us to keep it to contact you about eligibility changes for this study or future Arbor Biotechnologies studies. The information we collect about you will not be shared with Arbor Biotechnologies or Worldwide Clinical Trials in any way that could identify you. Only aggregated data will be shared with Arbor Biotechnologies and Worldwide Clinical Trials.
You may withdraw your consent at any time and upon withdrawal of your consent we will promptly delete your personal data, except to the extent required to comply with our legal obligations.
You have the right of access and obtain copies of the personal data held by myTomorrows or Sponsor at any time. You also have the right to request the rectification or erasure of your personal data held by myTomorrows or Sponsor. If you wish to withdraw your consent or exercise any of these rights, contact myTomorrows at dataprotection@mytomorrows.com who will advise you of the appropriate process to do so. If you believe your personal information has not or is not being processed in accordance with the applicable regulations, you have the right to lodge a complaint with a supervisory authority. In this case, myTomorrows is obligated to provide you with the appropriate contact information for you to make such a complaint.

