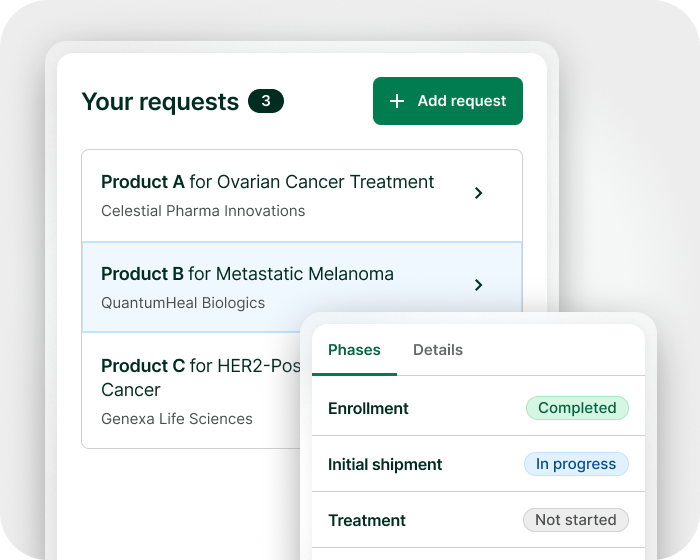
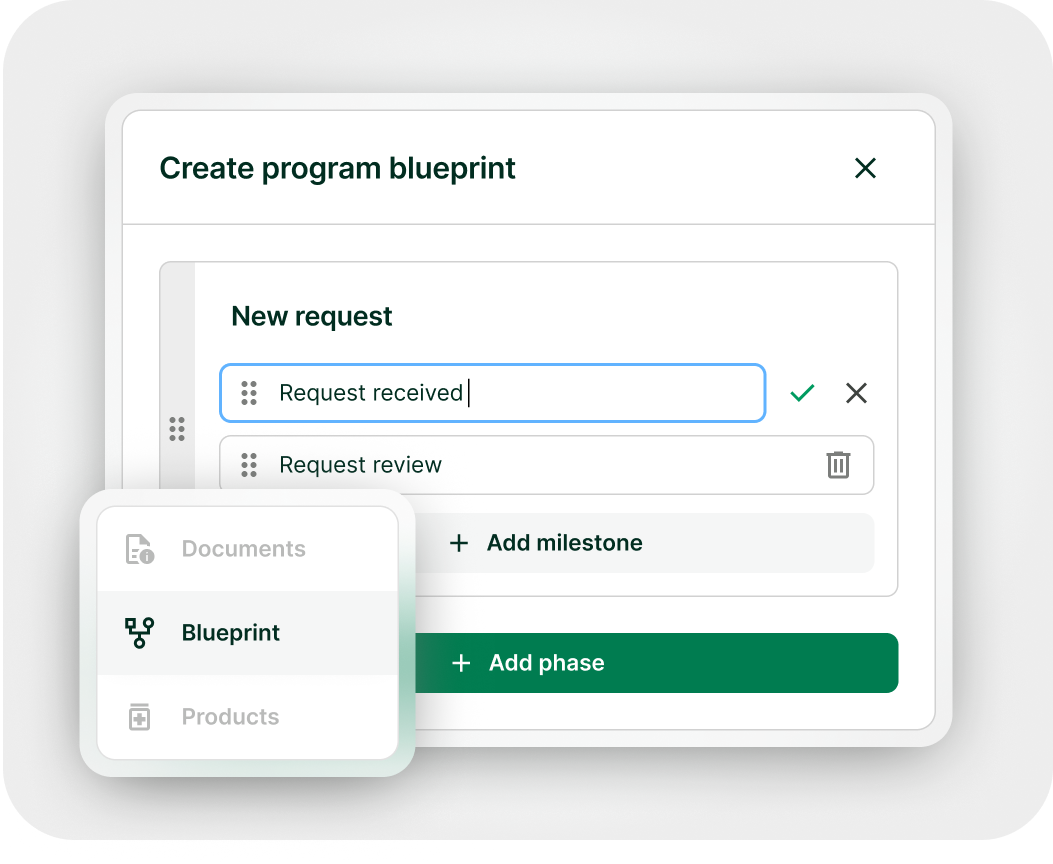
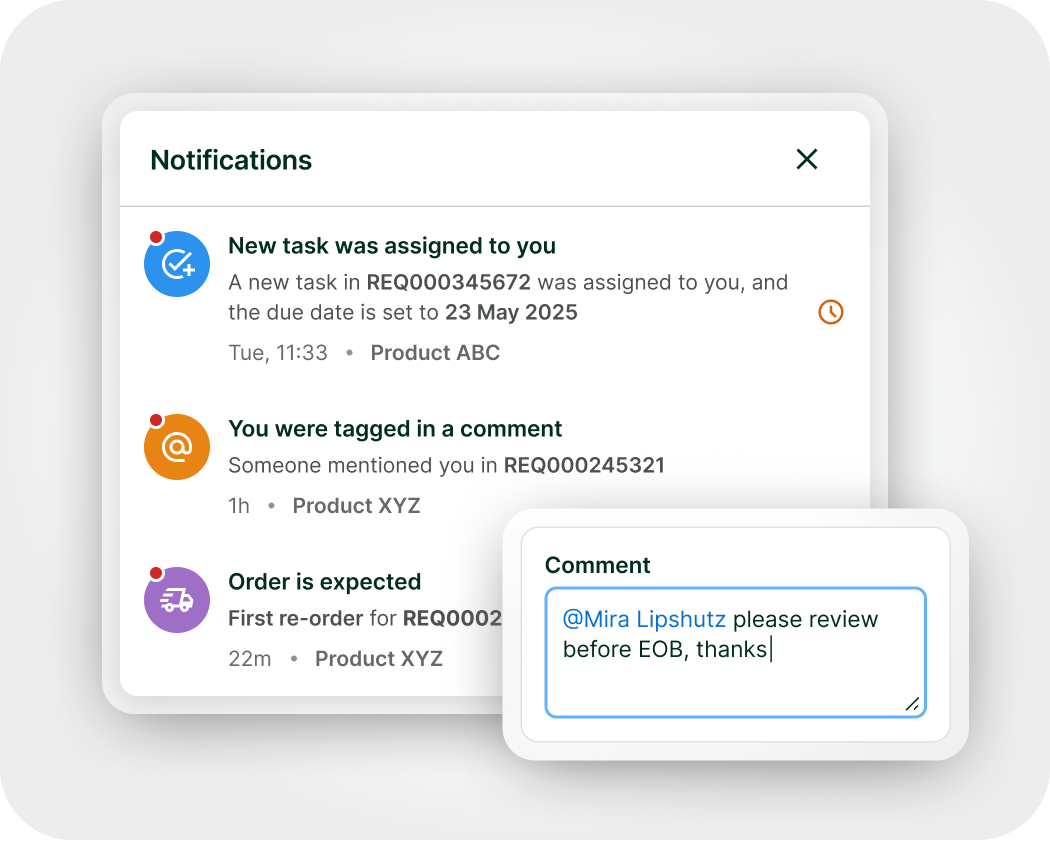
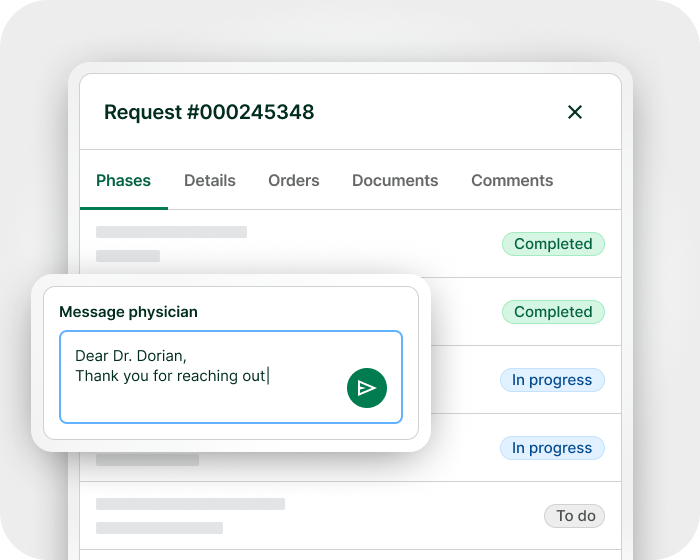
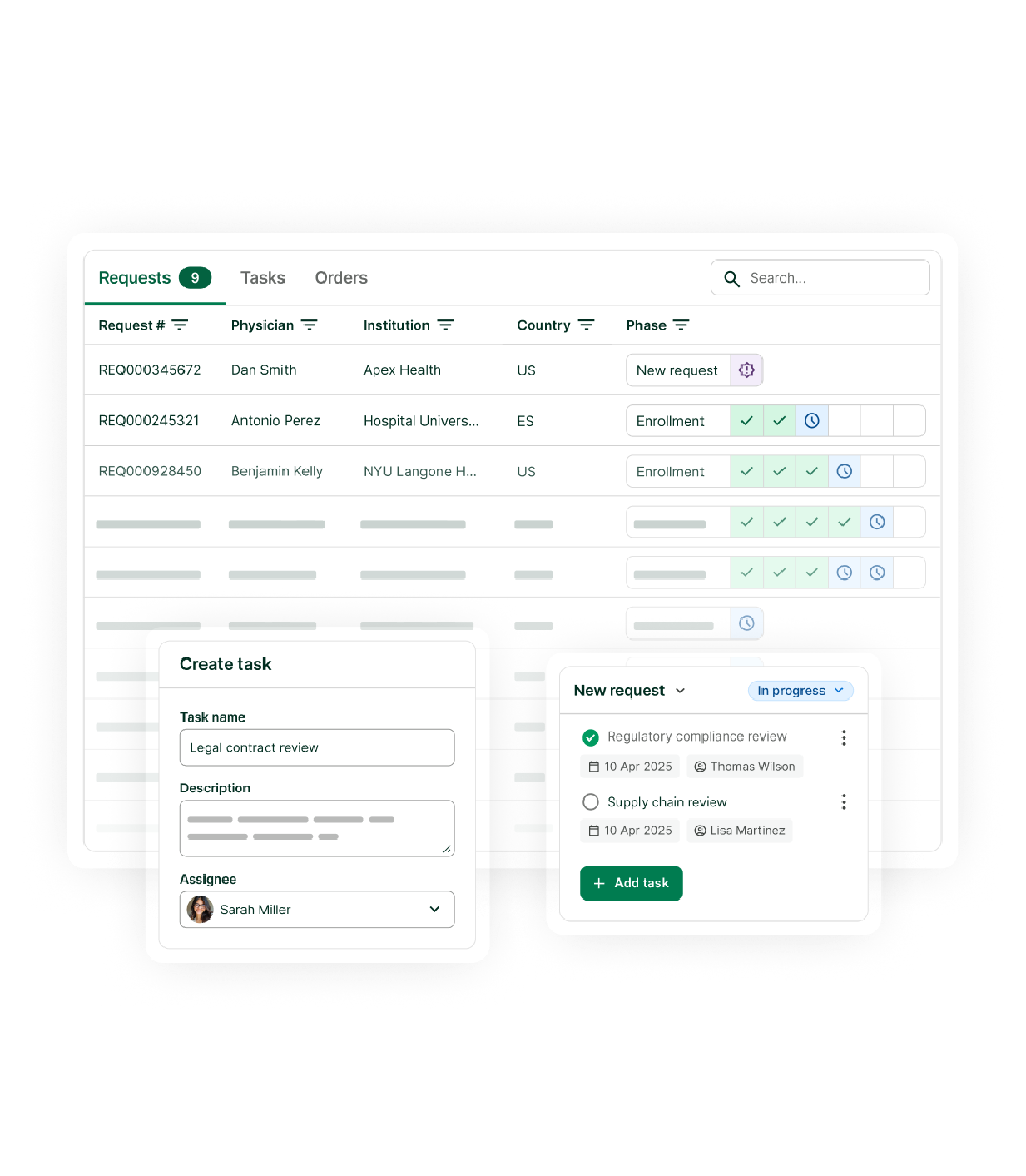
Expanded access doesn’t have to be complicated
From request management to real-world data collection, manage with clarity and control – all in one platform.


Warning: Trying to access array offset on value of type null in /var/www/html/wp-content/themes/custom/acf-flexible/section-part/header_–_left_content_right_image_patient.php on line 18
Warning: Trying to access array offset on value of type null in /var/www/html/wp-content/themes/custom/acf-flexible/section-part/header_–_left_content_right_image_patient.php on line 22