Expanded access doesn’t have to be complicated
From request management to real-world data collection, manage with clarity and control – all in one platform.
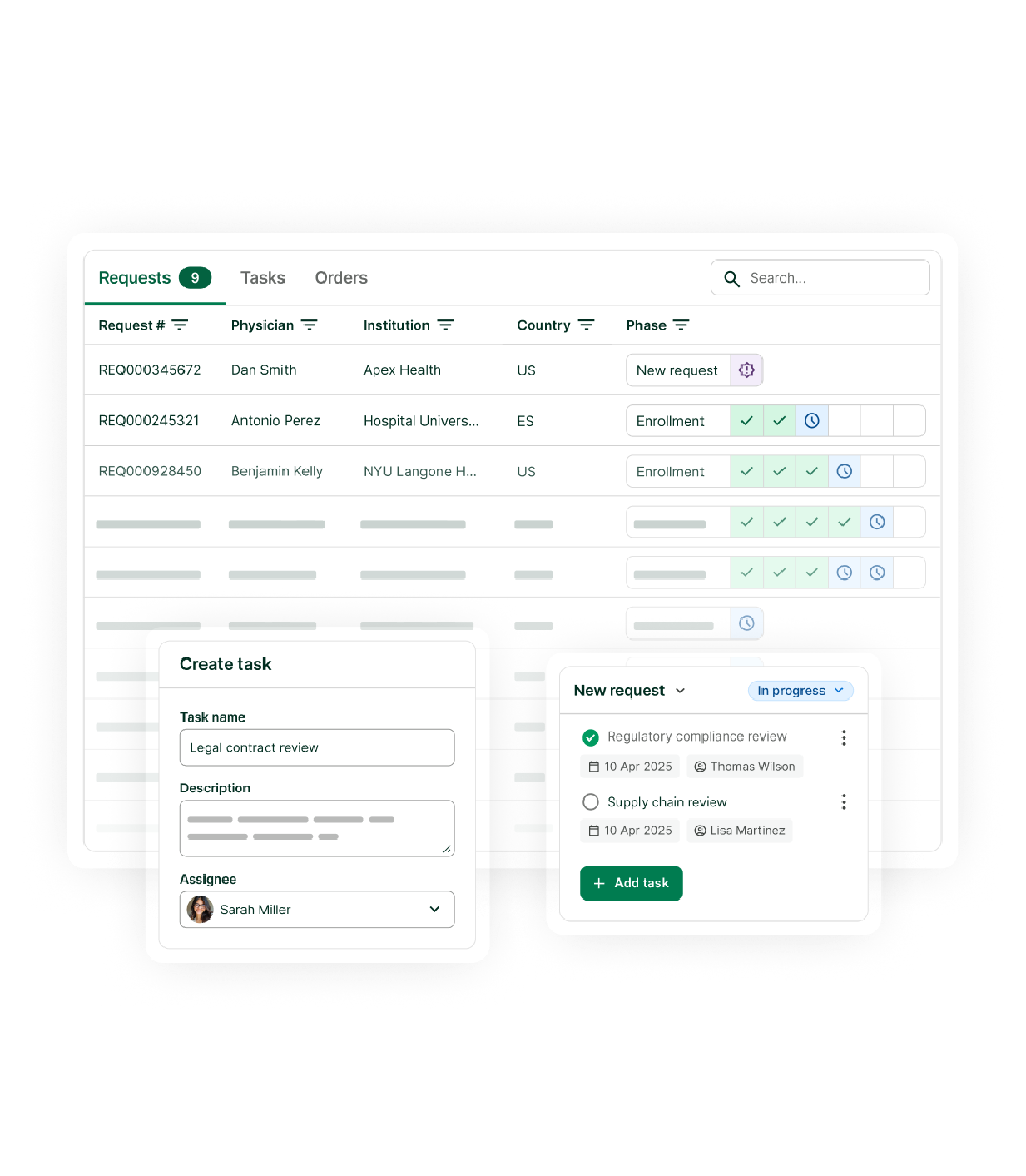


From request management to real-world data collection, manage with clarity and control – all in one platform.



Efficiently collect, triage and track all expanded access requests worldwide in a single, easy-to-use, secure platform. Manage documents, communicate with physicians, and track program performance: all in one integrated experience – ensuring centralized governance and program transparency.
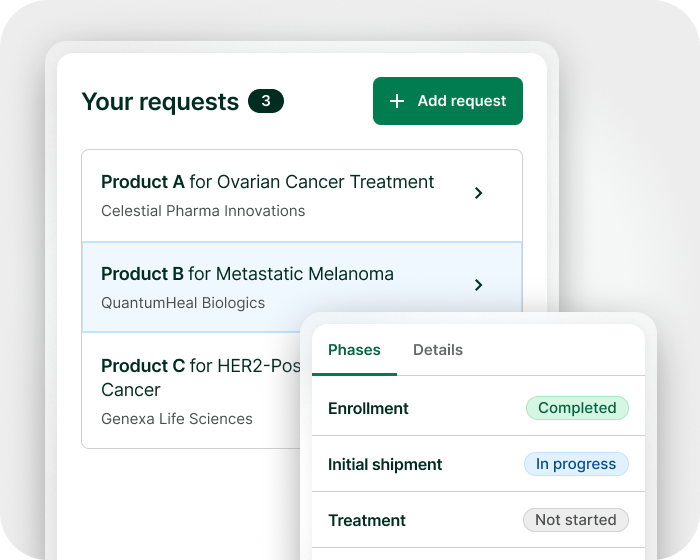
You are in the driver’s seat, from processing requests to approval and beyond. Create new programs, tailor workflows, owners, and forms with our intuitive tools – no engineers needed. Easily adapt to internal medical, legal, logistical and regulatory governance requirements. With audit-ready change history, you can log every action and configuration change for inspection.
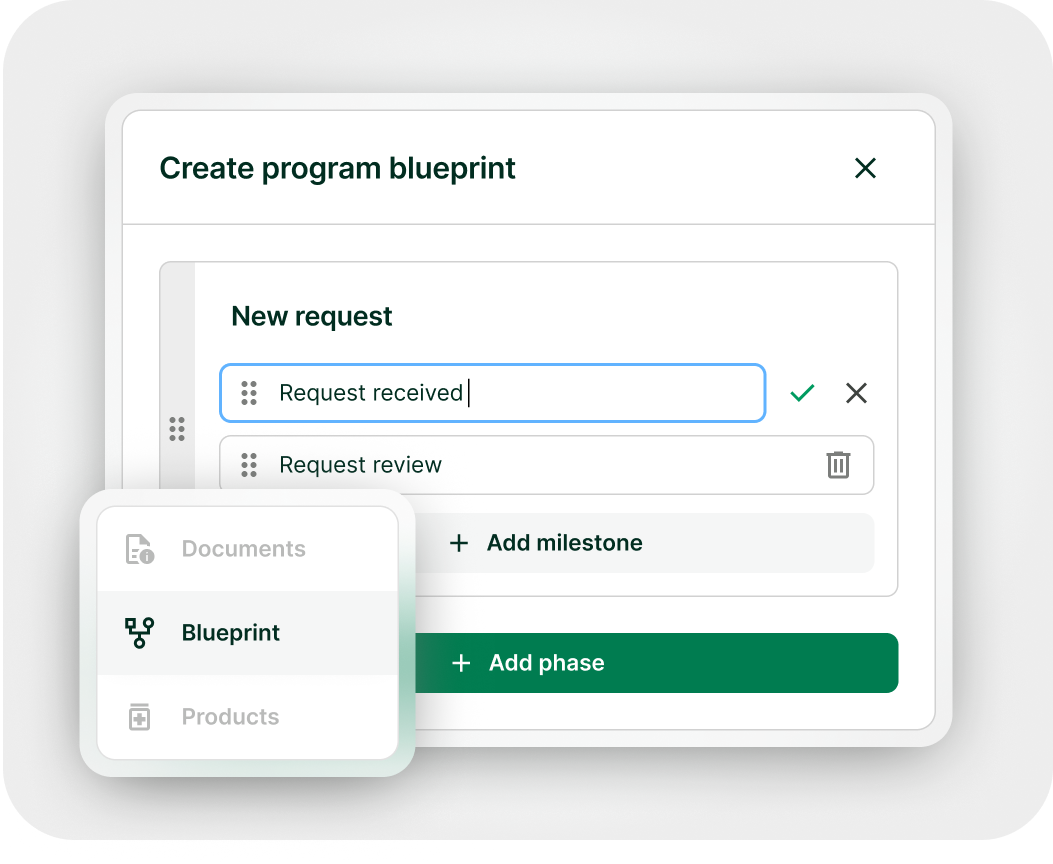
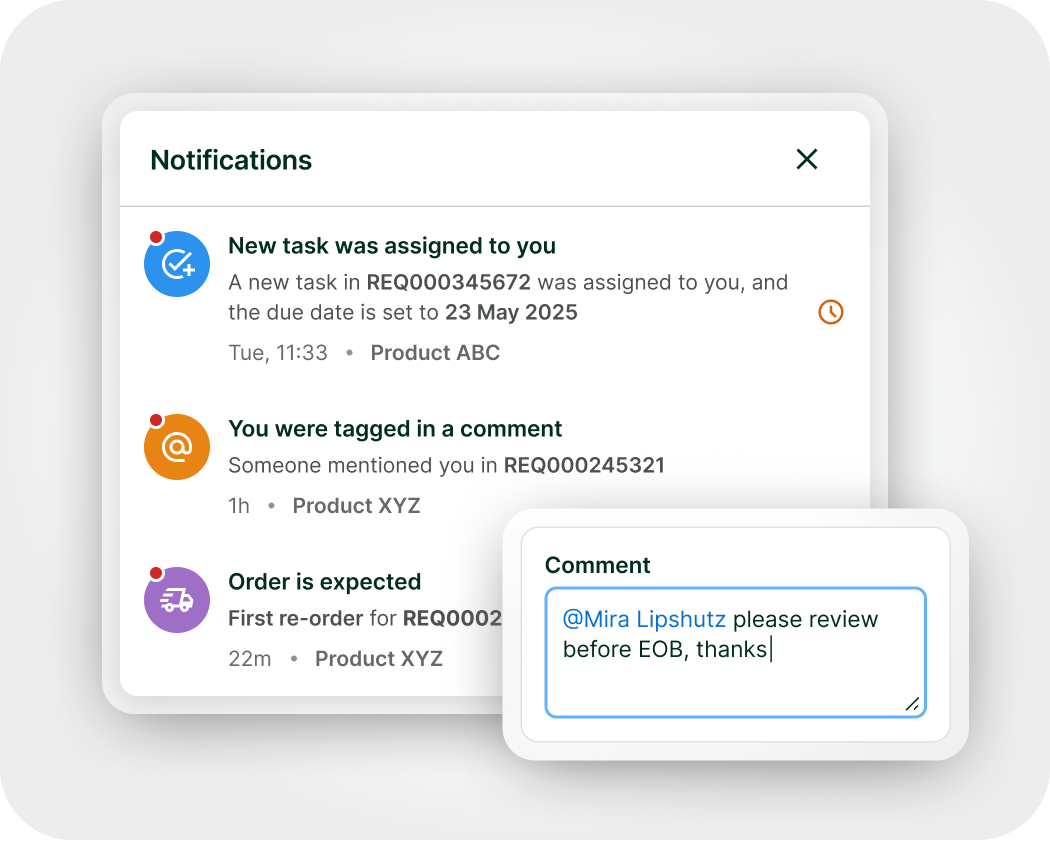
Stay aligned with your team and cross-functional partners. Collaborate, assign tasks and due dates with built-in notifications and reminders to keep milestone on track. Role-based access control ensures collaborators only see the data and actions relevant to their role for clear accountability and data security.
Turn your expanded access programs (EAPs) into a strategic asset by collecting real-world data (RWD) through Electronic Data Capture (EDC) system integration to support drug development, regulatory, and payor discussions. Leverage real-time data insights from configurable dashboards to optimize program operations.

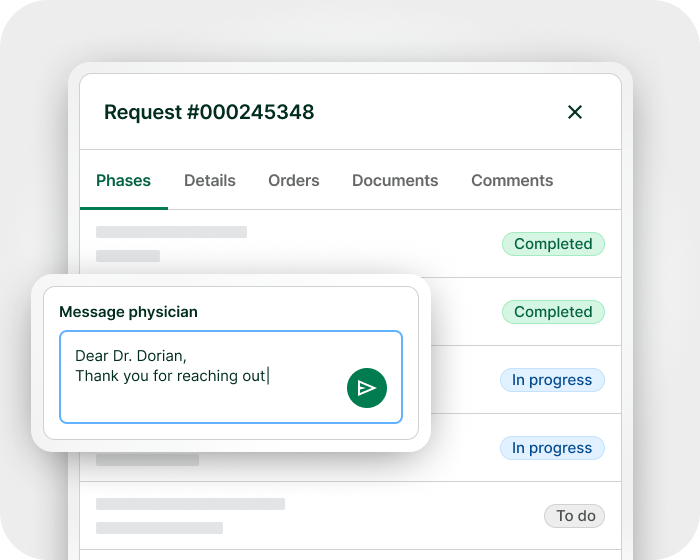
Communicate with physicians and receive medical documents via secure, encrypted two-way messaging. Physicians can easily submit a complete request from any device in less than 10 minutes. Keep physicians informed with live case status timelines and proactive updates. Built-in context‑aware FAQ and chatbot, as well as myTomorrows specialists provide expert guidance to physicians.
For over a decade, we’ve managed EAPs for more than 50 BioPharma companies worldwide across diverse therapeutic areas. Our purpose-built platform blends our deep regulatory expertise with intuitive technology – so you can simplify your expanded access operations with confidence.
Unlike solutions that address only narrow components of expanded access, our platform empowers you to manage and scale your programs – from single requests to global initiatives – with greater speed, compliance, and control from end-to-end.
With our intuitive and configurable tools you can effortlessly set up new programs, define workflows, manage user access, customize reporting and so much more – all by yourself. No code, no developers needed. We are always on-hand for complex support.
Managing expanded access programs is highly resource-intensive and fraught with operational and ethical complexities – from navigating regulatory approvals to aligning with many internal governance requirements. Whether you are managing a single ad-hoc request, or a global initiative, our intuitive and configurable platform puts you firmly in control, cuts administrative burden, improves operational transparency, and most importantly accelerate patient access, compliantly.


Trusted by
Enrolled
Across
Optimize your recruitment efforts with our patient-centric, multi-stakeholder solutions from clinical trial awareness and patient eligibility pre-screening to secure site referrals and enrollment
Backed by over a decade of experience in expanded access programs management, we support the design, execution, and close-out of expanded access programs (EAPs), tailored to fit any clinical program and market access strategy.
Our expert team provides full-service support for real-world data (RWD) collection and analysis in expanded access programs (EAPs) as well as strategic consultancy to help you meet your RWD objectives.