Expanded access (EA), can be a lifeline for patients with serious or life-threatening conditions who have exhausted all treatment and trial options. Healthcare professionals (HCPs) must navigate fragmented, unclear pathways to access, while BioPharma companies face operational, regulatory, and logistical challenges.
Recent regulatory shifts, including the 21st Century Cures Act1, highlight the urgency for EA policy disclosure. Though not mandated to provide access, sponsors who do so gain strategic benefits, such as gaining real-world insights into clinical use, stronger physician engagement, improved market-launch readiness, and, most importantly, demonstrate a commitment to patients and the healthcare community. However, many organizations still rely on fragmented, manual processes, leaving them vulnerable to inefficiencies and compliance risks.
Managing expanded access involves navigating a myriad of complex regulatory, compliance, and operational requirements. A program may begin small, but requests may grow unpredictably, placing increased pressure on operational teams to manage high-touch processes involving Medical Affairs, Regulatory, Legal & Compliance, Supply Chain, Pharmacovigilance, and even regional affiliates. Each plays a critical role, ensuring patient safety and maintaining compliance. However, coordinating & aligning across stakeholders is no easy task, especially when global requirements vary, and internal priorities diverge.
Each expanded access request can trigger a cascade of tasks, dependencies, and compliance risks if not managed through a centralized, responsive system. Yet most solutions cover only narrow functions, leaving cross-functional teams disconnected and overwhelmed. These challenges escalate when ad-hoc, single-patient requests arrive without clear protocols, leading to delays, inefficiencies, and risk of non-compliance.
Siloed tools also lack the ability to support real-time collaboration, evaluate requests at scale or generate aggregate reporting that inform executive insights. The result: companies face growing unpredictability, operational burden2, internal misalignment, and missed opportunities.
Unlike general-purpose systems, our Expanded Access platform for BioPharma was built from the ground up, shaped by direct execution and insights gained from the field. It reflects how teams work under pressure across functions, timelines, and geographies. Where we see gaps and barriers, we incorporated solutions to address them. Without a purpose-built solution informed by real-world challenges, expanded access risks becoming a bottleneck and an organizational burden, potentially delaying patient access, with reputational risk.
To enhance clarity and alignment, there is a need for a unified platform that serves as a one-stop shop for all stakeholders. This includes physicians as well as global and local BioPharma teams, across all types and variations of EA requests, from structured EAPs to ad hoc requests. myTomorrows’ platform offers a single solution to manage requests across any drug development with built-in capabilities for consolidated reporting, governance, and visibility. This enables executive leadership to assess program value while ensuring consistency and compliance across regions and functions.
At the heart of the platform, is a structured and robust request intake and case management system, forming the backbone of the EA request lifecycle. This ensures transparency for treating physicians, and enables seamless communication from initial physician request to closeout.
For BioPharma companies, everything starts with effective request capture management, the foundational step in transforming expanded access operations. By streamlining how requests are submitted, tracked, and triaged, organizations can reduce unnecessary delays, minimize errors and non-compliance, and ensure that no opportunity for patient access is missed. The result is a scalable, agile, and impactful approach to EA management for all stakeholders involved.
Since inception, myTomorrows was founded with a mission to simplify the process for all stakeholders – BioPharma, physicians, patients and sites. Drawing on real-world operational experience from global EA programs (EAPs) across 128 countries, we have developed an end-to-end digital platform covering the full EA lifecycle: from setup to request capture and triage, to regulatory coordination, supply management & delivery, and real-world data collection.
Effective EAP management requires more than a few basic tools, it requires operational depth and regulatory intelligence translating evolving policies into dynamic and/or structured executable workflows.
Our platform solution is built in-house with a scalable architecture designed for seamless integration with client systems and custom workflows. It supports real-time insights, analytics and role-based access, enabling teams to tailor user journeys to meet the specific needs of each program. This flexibility ensures that stakeholders can operate efficiently while maintaining control, compliance, and adaptability across diverse EA scenarios globally, aligning with regulatory and country specific requirements.
Unlike other solutions, the myTomorrows’ platform is shaped by the operational realities of expanded access delivery. Our team has taken everything we have learned running complex programs across the globe and embedded it into our platform design, ensuring it reflects how teams actually work under pressure, across functions, and vastly different geographies. This operational depth translates into meaningful business impact: fewer manual handoffs, faster regulatory alignment, and more strategic, case-driven approaches.
Key differentiators include:
As global access pathways evolve, the demand for intelligent, adaptable infrastructure has never been more urgent. Companies need flexible solutions that work standalone or with embedded service support. The myTomorrows platform meets this need by helping BioPharma companies, physicians, and sites work together to support patients when time and access matters the most.
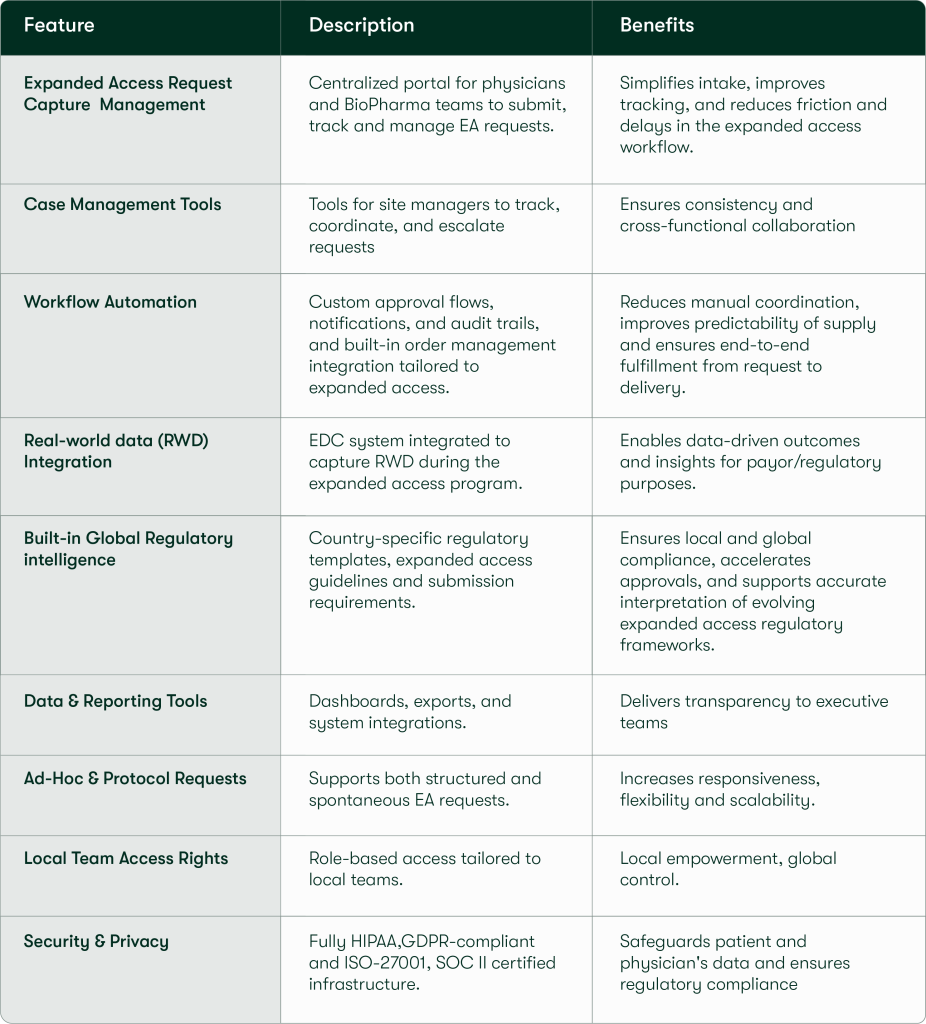
Our platform features include:
As drug development and expanded access continue to rapidly evolve, particularly with growing demands for long-term follow-up in rare diseases, the need to capture supportive RWD3 and monitor patient outcomes is more critical than ever. In addition, ethical frameworks like the Declaration of Helsinki now further underscore the importance of post-trial access4 treatment continuity, reinforcing the necessity of structured, long-term access.
Platform solutions play a central role in adapting to this evolution. They enable companies to develop a more complete picture of the treatment journey and clinical outcomes by supporting the collection of alternative or confirmatory data that can support regulatory and payer discussions. When these crucial insights are integrated across the drug development lifecycle, organizations are better equipped to adapt to emerging variables and reduce development costs by making smarter decisions, earlier.
As global access pathways expand and data expectations grow, BioPharma companies need more than just tools, they need a comprehensive and integrated approach. With myTomorrows, companies can flexibly combine technology and expanded access services as needed, whether to support high-volume global expanded access programs or more complex, resource-intensive requests. We offer both hybrid support models and standalone options, regardless – companies recognize the value of running their expanded access programs on a compliant platform.
With the launch of our purpose-built platform, myTomorrows is setting a new standard – and we are just getting started. Our next-generation AI capabilities will further transform how expanded access is managed and scaled. From regulatory intelligence trained on over a decade of expertise to intelligent task automation that cuts manual workload, these AI-integrated tools will further accelerate patient access and guide teams through complex global requirements.
With an intelligent AI-enabled end-to-end solution, BioPharma companies can turn expanded access into a scalable, compliant, and strategic capability that drives both broader goals and humanitarian impact.
Interested to learn how a platform solution can streamline your EA operations? Watch the webinar.
[1]https://mytomorrows.com/blog/BioPharma/why-it-is-important-develop-good-expanded-access-policy/
[2]mytomorrows.com/blog/biopharma/key-considerations-operationalizing-expanded-access-program/
[3]https://mytomorrows.com/blog/real-world-data/unlocking-the-potential-of-real-world-evidence-derived-from-expanded-access-programs/
[4]https://mytomorrows.com/blog/BioPharma/post-trial-access-treatment-how-expanded-access-may-offer-strategic-solution/
Dennis Akkaya 28 May 2025