more articles
view all blogsThe Role of Physical Therapy in ALS Care
Andrea Enguita
14 Jan 2026
16 mins read
Sentynl Selects myTomorrows to Lead Managed Access Program
myTomorrows Team
15 Dec 2025
4 mins read
Whitepaper: Advancing Neuro-Oncology Clinical Trial Access
Daniël Groeneweg
27 Nov 2025
8 mins read
End-Stage Parkinson’s Disease: Symptoms, Care, and Support
Andrea Enguita
18 Nov 2025
21 mins read
Getting Started: myTomorrows Referral Platform for Site Staff
Adrianne Rivard
14 Nov 2025
10 mins read
Our next chapter: €25M growth investment to scale patient access worldwide
Michel van Harten
11 Nov 2025
7 mins read
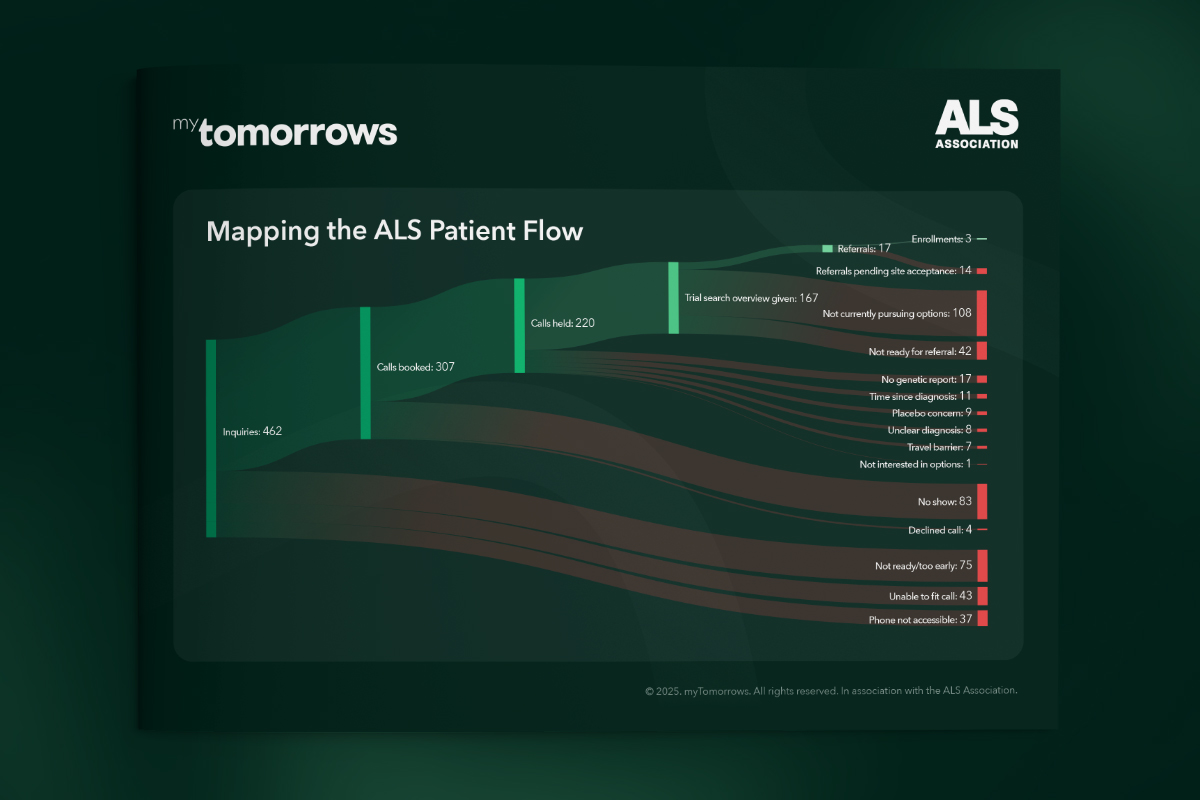
Accelerating ALS Clinical Trial Recruitment in the U.S.
Dennis Akkaya
10 Oct 2025
12 mins read
myTomorrows’ COO Vanessa Lemarié Named in Sifted’s Top 100 Women in Tech Europe
Michel van Harten
10 Oct 2025
2 mins read
Smarter Clinical Trial Recruitment that Delivers Faster Site Enrollment
Dennis Akkaya
18 Sep 2025
10 mins read
Exploring the Symptoms of Multiple System Atrophy
Andrea Enguita
13 Aug 2025
15 mins read