Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a rare, progressive neurological disorder that causes the degeneration of motor neurons controlling voluntary muscle movement. It often presents with muscle weakness and may involve difficulties with speaking, swallowing, or breathing, and it typically progresses to paralysis and respiratory failure, although the rate and pattern of progression can vary between patients. Despite decades of clinical research, there is currently no cure for ALS, and treatment options remain limited, with an average survival window of just 3 to 5 years after diagnosis.1 ALS occurs in two main forms: sporadic ALS, which accounts for approximately 90–95% of cases and arises without a known family history, and familial ALS, which makes up about 5–10% of cases and is inherited through genetic mutations.2
Faced with such urgent need, the ALS community has become a powerful force for progress. Patient advocacy groups like as well as ALS researchers and caregivers, have been highly successful in raising public awareness, funding critical ALS research, and driving major policy changes. A landmark achievement has been the Accelerating Access to Critical Therapies for ALS Act (Act for ALS), which continues to transform the ALS research landscape by making it easier to launch new ALS clinical trials and facilitating increased use of expanded access programs (EAPs) for eligible patients. These advocacy efforts have reshaped both ALS research and policy, echoing similar breakthroughs seen in other rare diseases such as Duchenne Muscular Dystrophy, where unified patient voices and targeted legislation accelerated innovation.
Clinical trials represent critical opportunities for people living with ALS, not only as a pathway to experimental therapies that may offer potential benefit, but also as a means of actively contributing to the advancement of ALS research. These trials drive innovation for a disease that continues to defy conventional therapeutic approaches.
As the scientific understanding of amyotrophic lateral sclerosis evolves, research is increasingly shifting from broad, symptom-based therapies to precision therapies that leverage genetics, biomarkers, and molecular pathways to target specific ALS subtypes at different stages of disease progression. Novel approaches include antisense oligonucleotides (ASOs) that silence disease-causing genes,3 nanomedicine techniques that use nanoparticles for targeted drug delivery and enhanced cellular uptake,4 and immunomodulatory therapies aimed at reducing neuroinflammation.5
The FDA granted accelerated approval in 2023 to tofersen (Qalsody)* for adults with ALS associated with a mutation in the SOD1 gene. Mutations in this gene lead to production of a toxic form of the SOD1 protein, and tofersen acts by reducing SOD1 protein levels in the cerebrospinal fluid.6 As an ASO therapy targeting a specific genetic mutation, tofersen represents an opportunity to more effective, personalized ALS treatments, but their highly targeted nature often limits eligibility, narrowing the number of trials accessible to each patient. This challenge is not unique to ALS. Across the rare disease landscape, trial specificity is limiting patients’ ability to participate in research, compounding the need for more inclusive trials and broader treatment access pathways. Fortunately, patient-centered trial designs, like those that reduce travel, offer more home-based visits, and limit the use of placebo controls, are increasingly being adopted, with support from regulators.7
Information about active ALS clinical trials recruiting in the U.S. can be found on ClinicalTrials.gov, a database maintained by the U.S. National Library of Medicine. myTomorrows also provides an AI-powered searchable database that indexes trial information from ClinicalTrials.gov. 8
*The mention of tofersen (Qalsody) is for informational purpose only. myTomorrows is not affiliated with, does not endorse, and receives no compensation from the manufacturer of this product
Expanded Access (EA) offers a pathway for people living with ALS who fall outside of the strict eligibility criteria of clinical trials or who do not reside near a clinical trial site. Expanded Access Programs (EAPs) typically allow a wider range of patients with serious or life-threatening conditions to access experimental products outside of the setting of clinical trials. The Act for ALS provides funding for sponsors to run EAPs, with a requirement to collect real-world data (RWD) on safety and efficacy. While different from clinical trial data, RWD is increasingly used in regulatory review and reimbursement discussions, particularly in rare diseases like ALS.9
EA can provide a bridge to treatment for patients who are otherwise excluded from research, while also offering opportunities to learn from RWD in populations under- or un-represented in trials. EAPs may face many of the same geographic and/or logistical hurdles as clinical trials, however, underscoring the ongoing need for stronger coordination, clearer pathways, and more equitable access across the clinical trial landscape.
The ALS community has embraced collaborative efforts to accelerate the testing of new interventions and promote rapid data sharing. These multi-site, multi-stakeholder initiatives are helping streamline ALS clinical trials, improving regulatory efficiency, and creating a more coordinated research environment.
At the center of ALS research are clinical trial sites, the operational backbone of ALS studies. In the United States, specialized multi-disciplinary ALS care centers, recognized by The ALS Association, play a critical role in patient care and often collaborate closely with clinical trial sites to facilitate patient referral and support throughout the clinical trial journey.10 These initiatives enhance coordination between patients, care teams, and clinical trial sites, significantly improve referral pathways, boost enrollment rates, and lead to better trial outcomes.11
An important methodological development in ALS research was the launch of the HEALEY ALS Platform Trial 12 (NCT04297683) in 2020. Platform trials enable simultaneous evaluation of multiple investigating treatments under a single, ongoing protocol with shared placebo controls, which can improve patient access to investigational therapies and potentially accelerate the development of potential ALS treatments.
Similarly, the TRICALs network, uniting more than 60 ALS centers across Europe, brings together patients, clinicians and researchers and leverages adaptive and predictive modeling to study various aspects of the disease at once.13 TRICALS has emerged as a leading example of international ALS research collaboration, frequently highlighted in scientific publications and rare disease trial networks.
Beyond the clinic, public awareness campaigns such as the ALS Ice Bucket Challenge14 dramatically increased visibility and funding, generating global momentum and unprecedented collaboration across the ALS community. Together, these advances show that coordinated efforts in research, advocacy, and public engagement are contributing to meaningful progress in the development of new treatments for people living with ALS.
Building on this strong foundation of collaboration and innovation, new partnerships are emerging to further expand patient access to investigational therapies and enhance data-driven insights. One such effort is the collaboration between The ALS Association and myTomorrows, which exemplifies how strategic alliances can bridge the gap between patients, clinicians, and the global research ecosystem.

With the ultimate goal to help make ALS a more livable disease, 15 myTomorrows and the ALS Association have partnered 16 to improve clinical trial navigation and for people living with ALS. This collaboration aims to reduce the barriers preventing patients from participating in clinical trials and enrich partner understanding of the ALS patient journey. At the core of this partnership is a dedicated patient navigation service that combines AI-driven trial pre-screening, personalized trial matching, and a comprehensive clinical trial database.17 myTomorrows patient navigators provide tailored support to help patients and caregivers explore clinical trial options that match their medical profile, overcome logistical and informational hurdles, and stay engaged throughout the process. At the core of this partnership is a dedicated patient navigation service that leverages myTomorrows’ AI-driven trial pre-screening to match patients’ medical profiles to clinical trials.18 In addition, the navigators provide tailored support to help patients and caregivers interpret clinical trial options that match their medical profile, overcome logistical and informational hurdles, and stay engaged throughout the process.

Since the myTomorrows & ALS Association collaboration in March 2024, more than 460 people living with ALS have accessed the patient navigation support to explore their potential clinical trial options. Early insights have revealed that some of the most critical learnings emerge from the points at which individuals disengage along the clinical trial journey, between initial exploration and trial enrollment. These drop-off points offer valuable opportunities to better understand the barriers faced–whether logistical, informational, or emotional—and to refine support strategies to ensure more seamless and sustained engagement throughout the process.
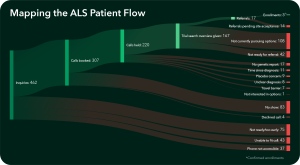
In order to contextualize user disengagement, a user feedback survey was initiated to help shed light on why drop offs occur at particular stages in the process. Key learnings, presented in a recent poster at the 2025 ALS Nexus Conference, 19 include:

The intent of the ALS collaboration is to overcome persistent challenges in ALS research, build stronger alignment across all stakeholders, including physicians, trial sponsors, clinical trial sites, navigation services, and patient advocacy groups, is essential. Clinical trial sites remain crucial, but their effectiveness is shaped by sponsor protocols and limited by capacity and resources. Strengthening coordination with site teams, particularly site coordinators, can streamline referrals and improve trial uptake.
While multidisciplinary ALS centers play a key role in care and research, many patients begin their journeys outside of these settings. Engaging community and general neurologists, secondary ALS clinics, and primary care providers is essential to reaching patients earlier, when they are more likely to be eligible, and to widening trial access to those outside of centralized networks.
Building on progress in other neuromuscular disease indications, myTomorrows is helping forge a stakeholder-aligned ALS referral ecosystem, one that creates smoother referral pathways, improves recruitment efficiency, and strengthens enrollment. Together with site-centric coordination and upstream efforts such as physician engagement, sponsor flexibility, and patient-centered navigation, barriers can be reduced, and research made more accessible. In alignment with the National Academies’ vision to make ALS a livable disease, these approaches shift ALS research toward a future where every person living with ALS has a fair chance to contribute to and benefit from clinical discovery.
[1] Mayo Clinic. Amyotrophic lateral sclerosis (ALS). https://www.mayoclinic.org/diseases-conditions/amyotrophic-lateral-sclerosis/symptoms-causes/syc-20354022
[2] ALS Association. Familial vs. Sporadic ALS. https://www.als.org/understanding-als/who-gets-als/familial
[3] Boros BD, Schoch KM, Kreple CJ, Miller TM. Antisense Oligonucleotides for the Study and Treatment of ALS. Neurotherapeutics. 2022;19(4):1145-1158. doi:10.1007/s13311-022-01247-2
[4] Vucic S, Menon P, Huynh W, et al. Efficacy and safety of CNM-Au8 in amyotrophic lateral sclerosis (RESCUE-ALS study): a phase 2, randomised, double-blind, placebo-controlled trial and open label extension. EClinicalMedicine. 2023;60:102036. Published 2023 Jun 8. doi:10.1016/j.eclinm.2023.102036
[5] Roemer SF, Ajami B, Dickson DW, Oskarsson B, et al. Study shows immune molecule may play key role in the progression of ALS. Proc Natl Acad Sci U S A. 2023. Accessed September 29, 2025. https://newsnetwork.mayoclinic.org/discussion/study-shows-immune-molecule-may-play-key-role-in-the-progression-of-als/
[6] Blair HA. Tofersen: First Approval. Drugs. 2023 Jul;83(11):1039-1043. doi: 10.1007/s40265-023-01904-6. PMID: 37316681.
[7] U.S. Food and Drug Administration. CDER Patient-Focused Drug Development. https://www.fda.gov/drugs/development-approval-process-drugs/cder-patient-focused-drug-development
[8] myTomorrows platform. https://platform.mytomorrows.com/search-results?condition=Amyotrophic%20Lateral%20Sclerosis&country=United%20States%20of%20America&utm_source=mytomorrows-landing&utm_content=search-widget
[9] Polak TB, Cucchi DGJ, van Rosmalen J, Uyl-de Groot CA, Darrow JJ. Generating Evidence from Expanded Access Use of Rare Disease Medicines: Challenges and Recommendations. Front Pharmacol. 2022;13:913567. Published 2022 May 23. doi:10.3389/fphar.2022.913567
[10] ALS Association. ALS Certified and Recognized Treatment Centers and Clinics. https://www.als.org/support/certified-centers-clinics
[11] Weemering, D. N., Beelen, A., Kliest, T., van Leeuwen, L. A. G., van den Berg, L. H., & van Eijk, R. P. A. (2024). Trial participation in neurodegenerative diseases: Barriers and facilitators—A systematic review and meta-analysis. Neurology, 103(1), e209503. https://doi.org/10.1212/WNL.0000000000209503
[12] ALS Association. ALS Association Makes Multi-Year Commitment to HEALEY ALS Platform Trial. https://www.als.org/stories-news/als-association-makes-multi-year-commitment-healey-als-platform-trial?
[13] van Eijk, R. P. A., Kliest, T., McDermott, C. J., Roes, K. C. B., Van Damme, P., Chio, A., … van den Berg, L. H. (2020). TRICALS: creating a highway toward a cure. Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, 21(7–8), 496–501. https://doi.org/10.1080/21678421.2020.1788092
[14] ALS Assocation. New Report Highlights Progress Made Because of ALS Ice Bucket Challenge. https://www.als.org/stories-news/new-report-highlights-progress-made-because-als-ice-bucket-challenge?
[15] National Academies of Sciences, Engineering, and Medicine. 2024. Living with ALS. Washington, DC: The National Academies Press. https://doi.org/10.17226/27739.
[16] ALS Association. myTomorrows Partners with the ALS Association to Support ALS Patients in Accessing Clinical Trials. https://www.als.org/stories-news/mytomorrows-partners-als-association-support-als-patients-accessing-clinical-trials
[17] myTomorrows. myTomorrows Partners with the ALS Association to Support ALS Patients in Accessing Clinical Trials. https://mytomorrows.com/blog/partnership/mytomorrows-partners-with-the-als-association-to-support-als-patients-in-accessing-clinical-trials/
[18] myTomorrows. myTomorrows launches an AI-powered clinical trial search tool. https://mytomorrows.com/blog/healthcare-professionals/mytomorrows-launches-an-ai-powered-clinical-trial-search-tool/
[19] myTomorrows. Poster: From Interest to Enrollment: Unlocking the ALS Clinical Trial Funnel. https://hsl.mytomorrows.com/hubfs/myTomorrows/NEALS%20poster.png
About the authors
Pre-approval Access Specialist at myTomorrows
John Massarelli is a Pre-approval Access Specialist at myTomorrows, where he focuses on the ethical and regulatory aspects of patient access to investigational drugs through clinical trials or expanded access programs. Prior to joining myTomorrows, John served as program coordinator for the Working Group on Compassionate Use & Preapproval Access (CUPA) at the NYU Grossman School of Medicine Division of Medical Ethics. He holds a Bachelor of Arts degree in philosophy from Fordham University.
Chief Commercial Officer of myTomorrows
Dennis Akkaya is the Chief Commercial Officer at myTomorrows, bringing over 20 years of experience in the BioPharma industry. He leads the company’s global commercial strategy, with a focus on accelerating patient access to treatments in development. Dennis has spent much of his career working with European biotech companies, developing deep expertise in pre-approval access programs, AI-powered clinical trial matching, and stakeholder engagement in rare disease research. A passionate advocate for the rare disease community, he regularly chairs and speaks at international events, driving thought leadership and raising awareness around unmet patient needs. Dennis holds an MSc in Finance.
Dennis Akkaya 10 Oct 2025