This article was updated in June 2025 by Andrea Enguita Marruedo, PhD (Medical Content Writer at myTomorrows) to include new insights and reflect the progress made in genetic testing for Duchenne Muscular Dystrophy since it was first published in 2022.
This blog is designed to provide information about the genetic causes of Duchenne Muscular Dystrophy (DMD) and the role of genetic testing in diagnosing the condition. It covers the different genetic mutations that can lead to Duchenne Muscular Dystrophy, how the condition is inherited, what genetic testing is, and the types of tests available—including options during pregnancy. It is intended for those living with Duchenne Muscular Dystrophy, their families, caregivers, and anyone seeking to understand how genetic testing works and how it can support early diagnosis and treatment planning.
Duchenne Muscular Dystrophy (DMD) is a genetic condition caused by different mutations (changes in the DNA) within the dystrophin gene, which is located on the X chromosome. This gene is responsible for producing the dystrophin protein, which plays an important role in keeping muscle cells intact. It acts like a shock absorber in muscle cells, helping to protect muscle fibers from getting damaged during contractions. Without enough dystrophin, muscles gradually become damaged and weaker.1
Duchenne Muscular Dystrophy mostly affects males, but in rare cases, females can be affected too. In Europe and North America, about 6 in every 100,000 people are living with the condition.1
Looking for more information about what causes Duchenne, how it’s diagnosed, and the signs to look for? Read our blog ‘Living with Duchenne Muscular Dystrophy: Treatments and Care Essentials’.
Duchenne Muscular Dystrophy can result from different mutations within the dystrophin gene, which is made up of 79 sections called exons. Having so many exons makes it one of the largest genes in the human body. Due to its large size, a wide range of mutations can occur along different parts of the gene. The most common types of dystrophin gene mutations that cause Duchenne include:
Depending on the specific mutation and how it affects dystrophin production, some of these changes can lead to a milder condition called Becker Muscular Dystrophy (BMD) instead of Duchenne. In Becker Muscular Dystrophy, the body still produces some dystrophin— just in a shorter, less effective form — so muscle weakening happens more slowly and is generally less severe.
If you want to know more about Becker Muscular Dystrophy, visit our blog ‘Navigating Becker Muscular Dystrophy: Symptoms, Diagnosis, and Treatment Options’.
Duchenne Muscular Dystrophy mostly affects males because the dystrophin gene is located on the X chromosome —one of the chromosomes that determines a person’s biological sex.
Humans have 23 pairs of chromosomes (46 in total), with one set inherited from each parent. These chromosomes carry genes, which means most genes come in pairs—one copy from each parent. 5, 6, 7 However, sex chromosomes are an exception:
Because males have only one X chromosome, they have only one copy of the genes located on it — such as the dystrophin gene — while females have two copies. 5, 7
Males will develop Duchenne Muscular Dystrophy if their X chromosome carries the mutation, as they lack a second X chromosome to compensate. Females have two X chromosomes, so if only one carries the mutation, the other usually provides enough dystrophin to prevent the disease. However, they can still pass the mutation on to their children (as “carriers”).1, 5, 8 Only around 10%-20% of female carriers of Duchenne Muscular Dystrophy exhibit symptoms of the disease, and these are typically milder than those observed in males, with a few exceptions.8, 9, 10
Duchenne Muscular Dystrophy is commonly inherited (passed down from a parent) (passed down from a parent). However, about one-third of cases result from spontaneous mutations in the dystrophin gene, meaning there is no family history of the condition. 11, 12, 13
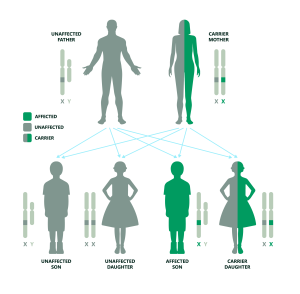
In most cases, Duchenne Muscular Dystrophy is passed down from a mother who is a carrier of the condition. This means she has one X chromosome with a mutation in the dystrophin gene, and one X chromosome without this mutation. Each of her children has a 50/50 chance of inheriting either X.
If the father is unaffected and the mother is a carrier of Duchenne Muscular Dystrophy:
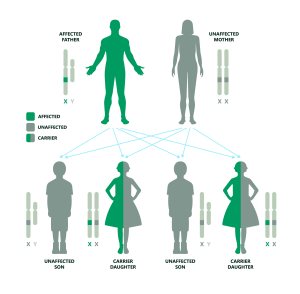
While this is highly unlikely due to the condition’s impact on mobility and life expectancy, in very rare cases a man with Duchenne may become a father. If the father is affected and the mother is unaffected and not a carrier:
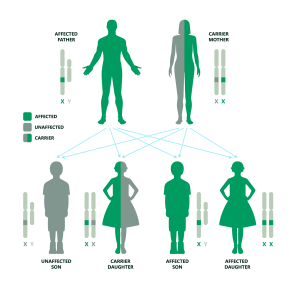
If the father is affected and the mother is a carrier:
These mutations happen randomly on the dystrophin gene, often very early after the egg is fertilized—before the baby even begins to develop. Because of this, the mutation ends up in every cell of the child’s body. 15, 16 Even though there’s no family history, once a child has this new mutation, it can be passed on to future generations. 1, 8, 14
Genetic testing, also known as DNA testing, is a type of medical test that looks for mutations in a person’s DNA.[17], [18] For Duchenne Muscular Dystrophy, genetic testing examines the dystrophin gene to detect the presence of a mutation and determine its specific type and location.1, 19, 20 It is usually performed on a blood or saliva sample and is currently the most accurate and widely accepted way to diagnose Duchenne. 1, 21
Genetic testing for Duchenne Muscular Dystrophy can:
There are several types of DNA genetic tests used to diagnose Duchenne Muscular Dystrophy. The type of test a doctor recommends often depends on whether a specific mutation in the dystrophin gene has already been identified in the family.
When a specific mutation has already been identified in a family member, the doctor may request familial mutation testing. This test looks only for that exact mutation, making it faster and easier than testing the entire gene. The exact testing method used depends on the type of mutation that was previously found in other members of the family. 19
In cases where no mutation has yet been identified, a step-by-step approach is usually followed:
A newer technology, called Next Generation sequencing, can detect deletions, duplications, and smaller point mutations in one single test. 19, 22, 23 While currently not yet available everywhere, it is increasingly being offered in specialized laboratories with access to advanced sequencing technology. 19
In a small number of cases (about 1–5%), no mutation is found even after thorough genetic testing with MLPA, CGH array testing, Sanger sequencing, or Next Generation Sequencing. 19, 24 This doesn’t always rule out Duchenne, and further testing may be needed to confirm the diagnosis. Follow-up tests may include:
For families with a history of Duchenne, prenatal genetic testing can assess whether a fetus carries the dystrophin gene mutation that causes the condition. 21, 25 Two main procedures are currently used to diagnose Duchenne during pregnancy:
Both procedures are generally considered safe but carry a small risk of miscarriage (the unintentional loss of a pregnancy), estimated to be between 0.25% and 1%. 25, 26, 27
A newer approach called non-invasive prenatal diagnosis (NIPD) is currently being developed for genetic conditions like Duchenne Muscular Dystrophy. This method involves collecting a simple blood sample from the mother, which contains small DNA fragments of the fetus that naturally circulate in her bloodstream. These fragments can be analyzed to detect a specific Duchenne gene mutation. [21], [26], [29] Because NIPD does not require a sample from the uterus, it avoids the small risk of miscarriage associated with invasive procedures like chorionic villus sampling (CVS) or amniocentesis. Early research suggests that NIPD has a high level of accuracy when the specific familial mutation is known. However, NIPD is not currently considered an emerging technology for Duchenne diagnosis. It is not yet part of standard prenatal screening, and further research is ongoing to better understand its accuracy, availability, and potential for wider clinical use. 21,26, 28
Prenatal genetic testing can have certain limitations. In some cases, results may be inconclusive, misleading, or unexpected. A normal result does not guarantee the baby will be free from all health conditions—it only confirms the absence of the specific muscular dystrophy mutation identified within the family. Furthermore, prenatal diagnosis can only be performed if there is a confirmed and clearly defined genetic diagnosis within the family. 21, 29
Duchenne Muscular Dystrophy (DMD) is a genetic condition caused by mutations in the dystrophin gene. Genetic testing helps confirm a Duchenne diagnosis, identify the type of dystrophin mutation causing the condition and guide treatment decisions. There are several types of genetic tests. If a known Duchenne mutation runs in the family, familial mutation testing can check for that exact genetic change. If no mutation has been identified yet, testing usually follows a step-by-step process: first looking for large deletions or duplications, then checking for smaller mutations. A newer test called Next Generation Sequencing (NGS) can detect all these mutation types in a single test, although it is available only in a few specialized laboratories. In some cases, if no mutation is found, additional tests—such as a muscle biopsy or RNA analysis—may be needed. For families with a known Duchenne mutation, prenatal testing during pregnancy (via chorionic villus sampling or amniocentesis) can check whether the baby has inherited a specific Duchenne mutation. A newer, non-invasive method called non-invasive prenatal diagnosis (NIPD), which uses a blood sample from the mother, is also being developed for conditions like Duchenne.
If you want to explore clinical trial options, you can book a call with a Patient Navigator to discover your options and learn more about clinical trials.
At myTomorrows, we have a team of Patient Navigators, who are multi-lingual professionals with a medical background, who can help you to explore your treatment options and support you through your journey.
[1] MDA, “Duchenne Muscular Dystrophy.” Accessed: Mar. 19, 2025. [Online]. Available: https://www.mda.org/disease/duchenne-muscular-dystrophy
[2] “Types of Genetic Variants – Parent Project Muscular Dystrophy.” Accessed: Apr. 01, 2025. [Online]. Available: https://www.parentprojectmd.org/about-duchenne/what-is-duchenne/types-of-genetic-variants/
[3] V. Venugopal and S. Pavlakis, “Duchenne Muscular Dystrophy,” Treasure Island (FL): StatPearls Publishing, Jul. 2023, Accessed: Apr. 01, 2025. [Online]. Available: https://www.ncbi.nlm.nih.gov/books/NBK482346/
[4] F. Muntoni, S. Torelli, and A. Ferlini, “Dystrophin and mutations: One gene, several proteins, multiple phenotypes,” Lancet Neurology, vol. 2, no. 12, pp. 731–740, Dec. 2003, doi: 10.1016/S1474-4422(03)00585-4.
[5] NHS, “Muscular dystrophy.” Accessed: Apr. 11, 2025. [Online]. Available: https://www.nhs.uk/conditions/muscular-dystrophy/
[6] National Human Genome Research Institute, “Diploid.” Accessed: Apr. 11, 2025. [Online]. Available: https://www.genome.gov/genetics-glossary/Diploid
[7] MedlinePlus Genetics, “How many chromosomes do people have?” Accessed: Apr. 11, 2025. [Online]. Available: https://medlineplus.gov/genetics/understanding/basics/howmanychromosomes/
[8] Parent Project Muscular Dystrophy, “Care For Carriers.” Accessed: Apr. 11, 2025. [Online]. Available: https://www.parentprojectmd.org/care/for-carriers/
[9] P. Riguzzi et al., “Deep characterization of females with heterozygous Duchenne muscular dystrophy mutations,” J Neurol, vol. 272, no. 3, p. 244, Mar. 2025, doi: 10.1007/S00415-025-12987-4.
[10] K. Bushby et al., “Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management,” Lancet Neurol, vol. 9, no. 1, pp. 77–93, Jan. 2010, doi: 10.1016/S1474-4422(09)70271-6.
[11] F. Fortunato, M. Farnè, and A. Ferlini, “The DMD gene and therapeutic approaches to restore dystrophin,” Neuromuscular Disorders, vol. 31, no. 10, pp. 1013–1020, Oct. 2021, doi: 10.1016/J.NMD.2021.08.004.
[12] T. Grimm, W. Kress, G. Meng, and C. R. Müller, “Risk assessment and genetic counseling in families with Duchenne muscular dystrophy.,” Acta Myol, vol. 31, no. 3, pp. 179–83, Dec. 2012, Accessed: Jan. 09, 2025. [Online]. Available: https://pmc.ncbi.nlm.nih.gov/articles/PMC3631803/
[13] D. Duan, N. Goemans, S. Takeda, E. Mercuri, and A. Aartsma-Rus, “Duchenne muscular dystrophy,” Nat Rev Dis Primers, vol. 7, no. 1, p. 13, Feb. 2021, doi: 10.1038/s41572-021-00248-3.
[14] “About Duchenne Muscular Dystrophy.” Accessed: Apr. 11, 2025. [Online]. Available: https://www.genome.gov/Genetic-Disorders/Duchenne-Muscular-Dystrophy
[15] “Genetic Causes – Parent Project Muscular Dystrophy.” Accessed: Apr. 17, 2025. [Online]. Available: https://www.parentprojectmd.org/about-duchenne/what-is-duchenne/genetic-causes/
[16] “Duchenne muscular dystrophy in detail | Duchenne UK.” Accessed: Apr. 17, 2025. [Online]. Available: https://www.duchenneuk.org/duchenne-in-detail/
[17] “Genetic Testing | Genomics and Your Health | CDC.” Accessed: Apr. 09, 2025. [Online]. Available: https://www.cdc.gov/genomics-and-health/counseling-testing/genetic-testing.html
[18] “What is genetic testing?: MedlinePlus Genetics.” Accessed: Apr. 09, 2025. [Online]. Available: https://medlineplus.gov/genetics/understanding/testing/genetictesting/
[19] Parent Project Muscular Dystrophy, “Is this Duchenne? Genetic Testing.” Accessed: Apr. 09, 2025. [Online]. Available: https://www.parentprojectmd.org/about-duchenne/is-it-duchenne/genetic-testing/
[20] “DMD Genetic Testing: 20 Things You Should Definitely Know – DMD Warrior.” Accessed: Apr. 09, 2025. [Online]. Available: https://dmdwarrior.com/dmd-genetic-testing/
[21] NHS, “Genetic testing – Muscular dystrophy.” Accessed: Apr. 09, 2025. [Online]. Available: https://www.nhs.uk/conditions/muscular-dystrophy/genetic-tests/
[22] A. Aartsma-Rus, I. B. Ginjaar, and K. Bushby, “The importance of genetic diagnosis for Duchenne muscular dystrophy,” J Med Genet, vol. 53, no. 3, p. 145, 2016, doi: 10.1136/JMEDGENET-2015-103387.
[23] M. Okubo et al., “Genetic diagnosis of Duchenne/Becker muscular dystrophy using next-generation sequencing: validation analysis of DMD mutations,” Journal of Human Genetics 2016 61:6, vol. 61, no. 6, pp. 483–489, Feb. 2016, doi: 10.1038/jhg.2016.7.
[24] A. Segarra-Casas et al., “Genetic diagnosis of Duchenne and Becker muscular dystrophy through mRNA analysis: new splicing events,” J Med Genet, vol. 60, no. 6, p. 615, Jun. 2022, doi: 10.1136/JMG-2022-108828.
[25] Muscular Dystrophy News Today, “Genetic Testing and Genetic Counseling.” Accessed: Apr. 16, 2025. [Online]. Available: https://musculardystrophynews.com/muscular-dystrophy-diagnosis/genetic-testing/
[26] M. Parks et al., “Non-invasive prenatal diagnosis of Duchenne and Becker muscular dystrophies by relative haplotype dosage,” Prenat Diagn, vol. 36, no. 4, pp. 312–320, Apr. 2016, doi: 10.1002/PD.4781.
[27] L. J. Salomon, A. Sotiriadis, C. B. Wulff, A. Odibo, and R. Akolekar, “Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis,” Ultrasound Obstet Gynecol, vol. 54, pp. 442–451, 2019, doi: 10.1002/uog.20353.
[28] L. Zaninović, M. Bašković, D. Ježek, and A. Katušić Bojanac, “Accuracy of Non-Invasive Prenatal Testing for Duchenne Muscular Dystrophy in Families at Risk: A Systematic Review,” Diagnostics, vol. 13, no. 2, p. 183, Jan. 2023, doi: 10.3390/DIAGNOSTICS13020183.
[29] “Reproductive Options – Parent Project Muscular Dystrophy.” Accessed: Apr. 16, 2025. [Online]. Available: https://www.parentprojectmd.org/care/for-carriers/reproductive-options/
Andrea Enguita Marruedo, PhD – Expert Medical Writer
Medical Content Writer at myTomorrows
Dr. Andrea Enguita Marruedo holds a master’s in Genetics and Cell Biology from the Autonomous University of Madrid and a PhD in Developmental Biology from the Erasmus Medical Centre in Rotterdam. She began her career in medical writing after completing her doctoral studies and has covered a wide range of therapeutic areas, including neuromuscular and neurodegenerative disorders, cancer, and diabetes.
Andrea specializes in translating complex biomedical topics into clear, accessible content for patients and healthcare professionals. With a strong research background and a passion for science communication, she is committed to delivering accurate, well-referenced content that supports greater awareness and understanding of medical topics among patients, caregivers, and clinicians.
myTomorrows Team 9 Dec 2022